When your child develops a persistent cough accompanied by breathing difficulties, distinguishing between croup and asthma becomes crucial for appropriate management. Both conditions affect the respiratory system but involve different anatomical regions and require distinct treatment approaches. Croup primarily affects the upper airway structures , including the larynx and trachea, whilst asthma involves the lower airways and bronchial passages. The characteristic barking cough of croup contrasts sharply with the wheezing patterns typical of asthmatic episodes. Understanding these fundamental differences enables parents and healthcare professionals to implement targeted interventions and prevent unnecessary complications. The timing, triggers, and acoustic characteristics of each condition provide valuable diagnostic clues that can guide immediate care decisions.
Croup pathophysiology: laryngotracheobronchitis and upper airway inflammation
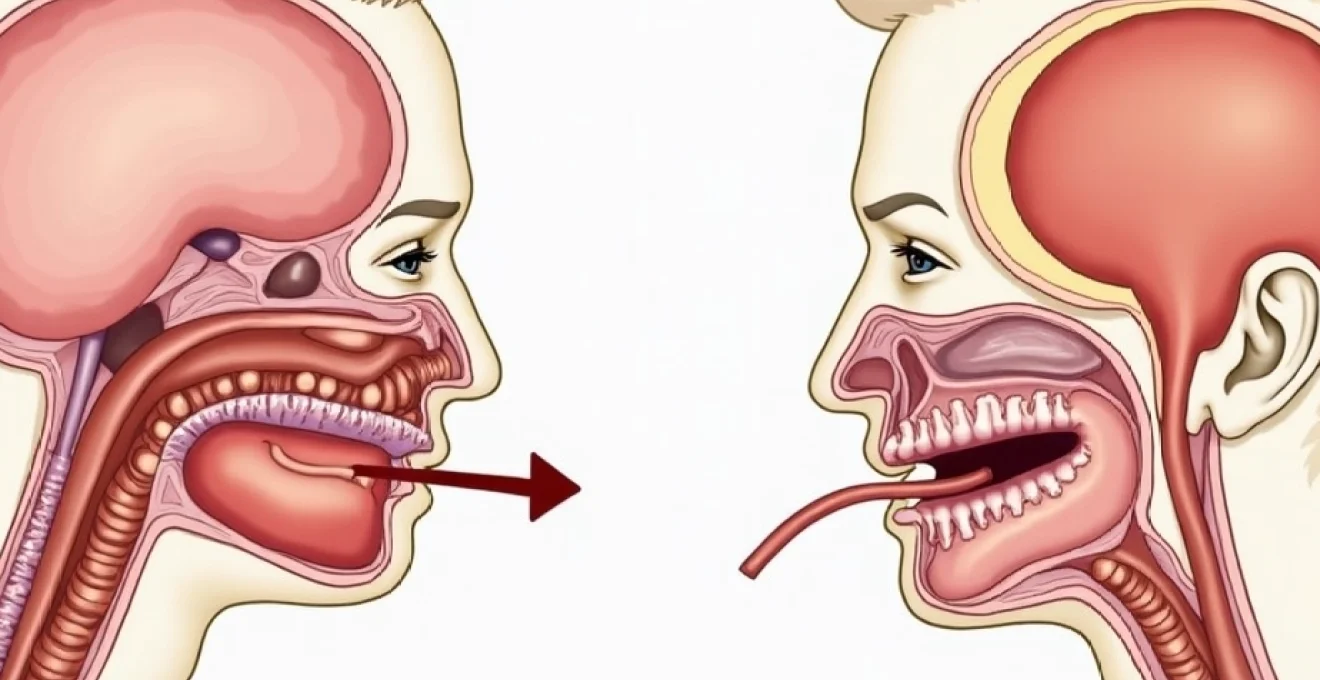
Croup represents a viral infection causing inflammation of the larynx, trachea, and bronchi, collectively termed laryngotracheobronchitis. This inflammatory process results in mucosal oedema and increased secretions within the upper respiratory tract. The narrowing of these critical airway structures creates the distinctive clinical presentation that differentiates croup from other respiratory conditions. The subglottic region becomes particularly susceptible to inflammatory changes , leading to the characteristic stridor and breathing difficulties observed in affected children.
Parainfluenza virus type 1 and primary viral aetiology
Parainfluenza virus type 1 accounts for approximately 75% of croup cases, making it the predominant causative agent. Other viral pathogens including respiratory syncytial virus, adenovirus, and human metapneumovirus contribute to the remaining cases. The seasonal distribution of croup correlates with viral circulation patterns, with peak incidence occurring during autumn and winter months. These viruses demonstrate particular tropism for the respiratory epithelium, initiating inflammatory cascades that compromise airway patency.
Subglottic stenosis and stridor development mechanisms
The anatomical vulnerability of the subglottic region stems from its position as the narrowest portion of the paediatric airway. Inflammatory oedema in this area produces disproportionate airway compromise compared to similar degrees of inflammation in larger airways. Even minimal swelling can reduce the effective airway diameter significantly , creating the conditions necessary for stridor development. The rigid cricoid cartilage surrounding this region prevents external expansion, forcing all accommodation to occur internally through further luminal narrowing.
Barking cough phonation through laryngeal oedema
The distinctive barking quality of croup cough results from altered phonation through inflamed and oedematous vocal cords. Normal vocal cord vibration becomes disrupted by mucosal swelling and increased secretions, producing the characteristic seal-like barking sound. This acoustic signature remains pathognomonic for croup and rarely occurs in other respiratory conditions. The harsh, metallic quality distinguishes it from the productive coughs associated with lower respiratory tract infections or the dry coughs common in asthmatic presentations.
Inspiratory stridor versus expiratory wheeze acoustic patterns
Stridor represents a high-pitched, musical sound occurring during inspiration when air flows through narrowed upper airways. This contrasts markedly with expiratory wheeze, which occurs during expiration as air passes through constricted lower airways. Understanding these acoustic differences provides immediate diagnostic information without requiring sophisticated equipment. Stridor typically becomes audible when airway diameter reduces by approximately 50%, indicating significant upper airway compromise. The timing and quality of these sounds offer crucial insights into the anatomical location and severity of respiratory obstruction.
Asthma pathophysiology: bronchial hyperresponsiveness and inflammatory cascades
Asthma represents a chronic inflammatory disorder of the airways characterised by variable airflow limitation and bronchial hyperresponsiveness. The pathophysiological mechanisms involve complex interactions between genetic predisposition, environmental triggers, and immune system dysfunction. Unlike croup’s acute viral aetiology , asthma develops through sustained inflammatory processes that remodel airway architecture over time. The condition affects approximately 10-15% of children globally, with prevalence continuing to increase in developed nations.
Type 2 helper T-Cell mediated allergic responses
The predominant asthma phenotype involves Type 2 helper T-cell (Th2) mediated allergic inflammation. These cells orchestrate immune responses by releasing cytokines including interleukin-4, interleukin-5, and interleukin-13. The cytokine cascade promotes IgE production, eosinophil recruitment, and mast cell activation throughout the bronchial tree. This inflammatory environment creates the foundation for the characteristic features of asthmatic airways including smooth muscle hypertrophy, mucus hypersecretion, and epithelial damage.
Smooth muscle bronchoconstriction and beta-2 receptor function
Airway smooth muscle cells in asthmatic patients demonstrate enhanced contractility and altered calcium handling mechanisms. Beta-2 adrenergic receptors, which normally mediate bronchodilation, become desensitised through chronic inflammation and repeated exposure to bronchodilator medications. This receptor dysfunction contributes to the progressive nature of asthmatic symptoms and explains why some patients develop tolerance to rescue medications. The smooth muscle layer also undergoes structural changes including hyperplasia and hypertrophy, further compromising airway calibre.
Eosinophilic inflammation and IgE-Mediated degranulation
Eosinophils play a central role in asthmatic inflammation through the release of cytotoxic proteins and inflammatory mediators. These cells accumulate in bronchial tissue and secretions, releasing major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin. IgE-mediated mast cell degranulation triggers immediate hypersensitivity reactions characterised by rapid onset bronchospasm, increased vascular permeability, and mucus secretion. The combination of these mechanisms produces the acute exacerbations that define asthmatic episodes.
Airway remodelling through goblet cell hyperplasia
Chronic asthmatic inflammation leads to permanent structural changes termed airway remodelling. Goblet cell hyperplasia results in excessive mucus production that contributes to airway obstruction and impaired clearance mechanisms. Subepithelial fibrosis develops through increased collagen deposition, creating fixed airway narrowing that responds poorly to bronchodilator therapy. These irreversible changes emphasise the importance of early intervention and consistent anti-inflammatory treatment to prevent long-term complications.
Peak expiratory flow rate variability in obstructive patterns
Asthmatic patients demonstrate characteristic variability in peak expiratory flow rates that reflects the dynamic nature of airway obstruction. Daily variations exceeding 20% suggest inadequate asthma control and increased risk of exacerbations. The obstructive pattern shows reduced expiratory flow rates with preserved or increased lung volumes, contrasting with the restrictive patterns seen in some upper airway disorders. Morning dips in peak flow correlate with circadian variations in inflammatory mediator release and cortisol production.
Clinical presentation differentiation: westley croup score versus asthma control test
Clinical assessment tools provide standardised methods for evaluating disease severity and guiding treatment decisions. The Westley Croup Score incorporates five parameters: level of consciousness, cyanosis, stridor, air entry, and retractions. Scores range from 0 to 17, with higher values indicating more severe disease. Mild croup typically scores 0-2 points , moderate croup 3-5 points, and severe croup 6 or more points. This scoring system helps clinicians determine appropriate intervention levels and admission criteria.
The Asthma Control Test evaluates symptom frequency, activity limitation, shortness of breath, nighttime awakening, and rescue medication use over the preceding four weeks. Scores below 20 indicate poorly controlled asthma requiring treatment adjustment. The test provides valuable insights into disease impact on daily functioning and helps identify patients at risk for exacerbations. Regular monitoring using standardised tools improves outcomes by facilitating timely interventions and treatment modifications.
The key to accurate diagnosis lies in recognising the distinctive temporal patterns and acoustic characteristics that differentiate upper airway obstruction from lower airway disease.
Age distribution patterns offer additional diagnostic clues, with croup predominantly affecting children between 6 months and 6 years, peaking at 1-2 years of age. Asthma can develop at any age but commonly presents during early childhood, with many cases manifesting before age 5. The seasonal variation differs significantly, with croup showing clear autumn and winter predominance, whilst asthma exacerbations may occur year-round with individual trigger-specific patterns.
Diagnostic imaging protocols: anteroposterior neck radiographs and steeple sign recognition
Radiographic evaluation provides valuable diagnostic information in cases where clinical presentation remains unclear or when complications are suspected. The anteroposterior neck radiograph represents the standard imaging approach for suspected croup cases. The pathognomonic steeple sign appears as symmetric narrowing of the subglottic airway , creating a church steeple-like configuration on the frontal view. This finding demonstrates sensitivity of approximately 50% but maintains high specificity when present.
Lateral neck radiographs may reveal additional findings including increased soft tissue density anterior to the cervical vertebrae and loss of the normal cervical lordosis. However, these changes lack specificity and can occur with various upper respiratory infections. The decision to obtain imaging should balance diagnostic benefit against potential delays in treatment, particularly in severely symptomatic children requiring immediate intervention.
Chest radiography plays a more prominent role in asthma evaluation, particularly during acute exacerbations when complications such as pneumothorax or pneumonia are suspected. Hyperinflation with flattened diaphragms and increased anteroposterior chest diameter characterise the typical asthmatic chest radiograph. Peribronchial thickening and increased lung markings may indicate chronic inflammatory changes, whilst areas of atelectasis suggest mucus plugging.
Pharmacological management algorithms: nebulised budesonide versus salbutamol administration
Corticosteroids form the cornerstone of croup treatment, with evidence supporting both systemic and nebulised administration routes. Nebulised budesonide demonstrates efficacy comparable to oral prednisolone whilst potentially reducing systemic side effects. The typical dosing regimen involves 2mg of nebulised budesonide administered through face mask or mouthpiece. Clinical improvement typically becomes apparent within 30-60 minutes , with peak effect occurring at 2-4 hours post-administration.
Oral prednisolone remains the most commonly prescribed corticosteroid for croup, with doses ranging from 1-2mg/kg (maximum 40mg) as a single dose. The oral route offers practical advantages including ease of administration and widespread availability. Dexamethasone represents an alternative option with longer half-life, potentially providing extended symptom relief with single-dose administration.
The rapid onset of corticosteroid effects in croup contrasts with the delayed anti-inflammatory benefits seen in asthma treatment, reflecting different underlying pathophysiological mechanisms.
Salbutamol and other short-acting beta-2 agonists serve as first-line rescue medications for acute asthma exacerbations. The standard nebulised dose for children involves 2.5-5mg administered every 20 minutes for three doses during the first hour of treatment. Metered-dose inhalers with spacer devices demonstrate equivalent efficacy to nebulised administration whilst offering practical advantages including portability and reduced treatment time.
Long-acting beta-2 agonists such as salmeterol and formoterol provide sustained bronchodilation for up to 12 hours but should never be used as monotherapy due to safety concerns. Combination inhalers containing both corticosteroids and long-acting bronchodilators offer convenience and improved compliance whilst ensuring appropriate anti-inflammatory coverage.
Emergency department triage: severe inspiratory stridor assessment and intubation considerations
Emergency department assessment begins with rapid evaluation of airway compromise severity and the need for immediate intervention. Severe inspiratory stridor audible at rest indicates significant airway narrowing requiring urgent medical attention. The presence of biphasic stridor suggests critical airway compromise with risk of complete obstruction. Additional concerning features include altered mental status, cyanosis, severe retractions, and inability to speak or cry.
Intubation considerations in croup patients require careful evaluation of risks versus benefits. The inflamed and oedematous upper airway increases the technical difficulty of endotracheal tube placement whilst simultaneously increasing the risk of complete airway obstruction. A smaller endotracheal tube size should be selected , typically 0.5-1.0mm smaller than age-appropriate calculations would suggest. Preparation for emergency surgical airway access becomes essential when intubation attempts fail.
Asthmatic patients presenting to emergency departments require systematic assessment using standardised severity scores such as the Paediatric Asthma Severity Score (PASS) or the Pulmonary Index Score (PIS). These tools incorporate parameters including respiratory rate, oxygen saturation, auscultatory findings, and accessory muscle use. Scores guide treatment intensity and disposition decisions, with higher scores indicating increased likelihood of admission requirements.
The management of severe asthmatic exacerbations involves escalating bronchodilator therapy combined with systemic corticosteroids. Nebulised ipratropium bromide provides additive bronchodilation when combined with salbutamol, particularly beneficial during the first hour of treatment. Magnesium sulphate administration shows promise as adjunctive therapy in severe cases, with evidence supporting both intravenous and nebulised routes. Continuous monitoring becomes essential to detect clinical deterioration and identify patients requiring intensive care support or mechanical ventilation.